Archives
In this issue of Jabbari et
In this issue of , Jabbari et al. followed up on hair growth observed in a patient under baricitinib therapy, also a JAK1/2 inhibitor and studied JAK inhibitor action in the C3H/HeJ graft-recipient mouse model of AA (). Under JAK inhibitor treatment, CD8+ cell infiltrates and MHC class I and II expressions were markedly reduced in C3H/HeJ mice grafted with alopecic skin, both in a preventive and a therapeutic setting. In addition, gene Wortmannin profiling using the Alopecia Areata Disease Activity Index (ALADIN) biomarker for response to treatment confirmed the assumed normalization of the IFN-gamma gene expression signature. The  approach of Jabbari et al. represents an excellent model for translational work, where a specific clinical observation made in a special patient is complemented with mechanistic work, which nicely allows to link macroscopic appearance, immunohistochemistry and gene expression profiles with the current idea of how JAK inhibitors could be beneficial in AA ().
However, all euphoria should not let forget that JAK inhibitors inhibit multiple pathogenic pathways simultaneously. A broad spectrum of side effects already limits their use in the different licensed indications. Similarly, clinical studies on response rates in larger groups of patients will have to take into account the heterogeneity among AA patients. Maintenance of hair growth and relapse rates after cessation of therapy will have to be monitored carefully. Also, we have to be very aware of the fact that despite its tremendous psychosocial burden, AA is a benign lifelong genetic predisposition, with one third of AA patients being affected before 30years of age. Safety aspects will have to be carefully considered especially in this young population. With this regard, the small molecule JAK inhibitors do not only offer advantages for effective oral delivery, but are also highly interesting candidate molecules for topical treatment. While overall penetration rates in skin especially via the transfollicular route should not be a problem, a focus of research should be put on ways to increase follicular penetration and reduce systemic absorption. Incorporation in particle-based formulations or even the design of functionalized nanocarriers capable of targeting inflammatory infiltrates along the hair follicle could be one option for future developments in AA management strategies. Such targeted delivery may help increase local drug concentration and efficacy with reduced systemic side-effects.
approach of Jabbari et al. represents an excellent model for translational work, where a specific clinical observation made in a special patient is complemented with mechanistic work, which nicely allows to link macroscopic appearance, immunohistochemistry and gene expression profiles with the current idea of how JAK inhibitors could be beneficial in AA ().
However, all euphoria should not let forget that JAK inhibitors inhibit multiple pathogenic pathways simultaneously. A broad spectrum of side effects already limits their use in the different licensed indications. Similarly, clinical studies on response rates in larger groups of patients will have to take into account the heterogeneity among AA patients. Maintenance of hair growth and relapse rates after cessation of therapy will have to be monitored carefully. Also, we have to be very aware of the fact that despite its tremendous psychosocial burden, AA is a benign lifelong genetic predisposition, with one third of AA patients being affected before 30years of age. Safety aspects will have to be carefully considered especially in this young population. With this regard, the small molecule JAK inhibitors do not only offer advantages for effective oral delivery, but are also highly interesting candidate molecules for topical treatment. While overall penetration rates in skin especially via the transfollicular route should not be a problem, a focus of research should be put on ways to increase follicular penetration and reduce systemic absorption. Incorporation in particle-based formulations or even the design of functionalized nanocarriers capable of targeting inflammatory infiltrates along the hair follicle could be one option for future developments in AA management strategies. Such targeted delivery may help increase local drug concentration and efficacy with reduced systemic side-effects.
Since the early 1980s, there has been clinical data demonstrating the effects of beta-adrenergic agonist on lipid levels. Terbutaline was shown to raise HDL-c levels in healthy subjects after just two weeks of treatment (). This data in conjunction with other trials which revealed that beta-blockers lowered HDL-c and raised LDL-c as well as triglycerides (), supported the hypothesis that stimulation of the beta-2 receptor could result in favorable lipid changes. found that albuterol administration was associated with favorable changes in the serum lipid profile with significant lowering of LDL-c and increases in HDL-c in a small human trial without marked impairment of glucose tolerance or its physiologic determinants (). of found that another selective beta-2 agonist, R-bambuterol also significantly lowered LDL-c in a relatively small healthy volunteer population (). Since the racemic mixture of bambuterol was not effective in modifying the lipid levels, this suggests that there may be inhibitory effects between R a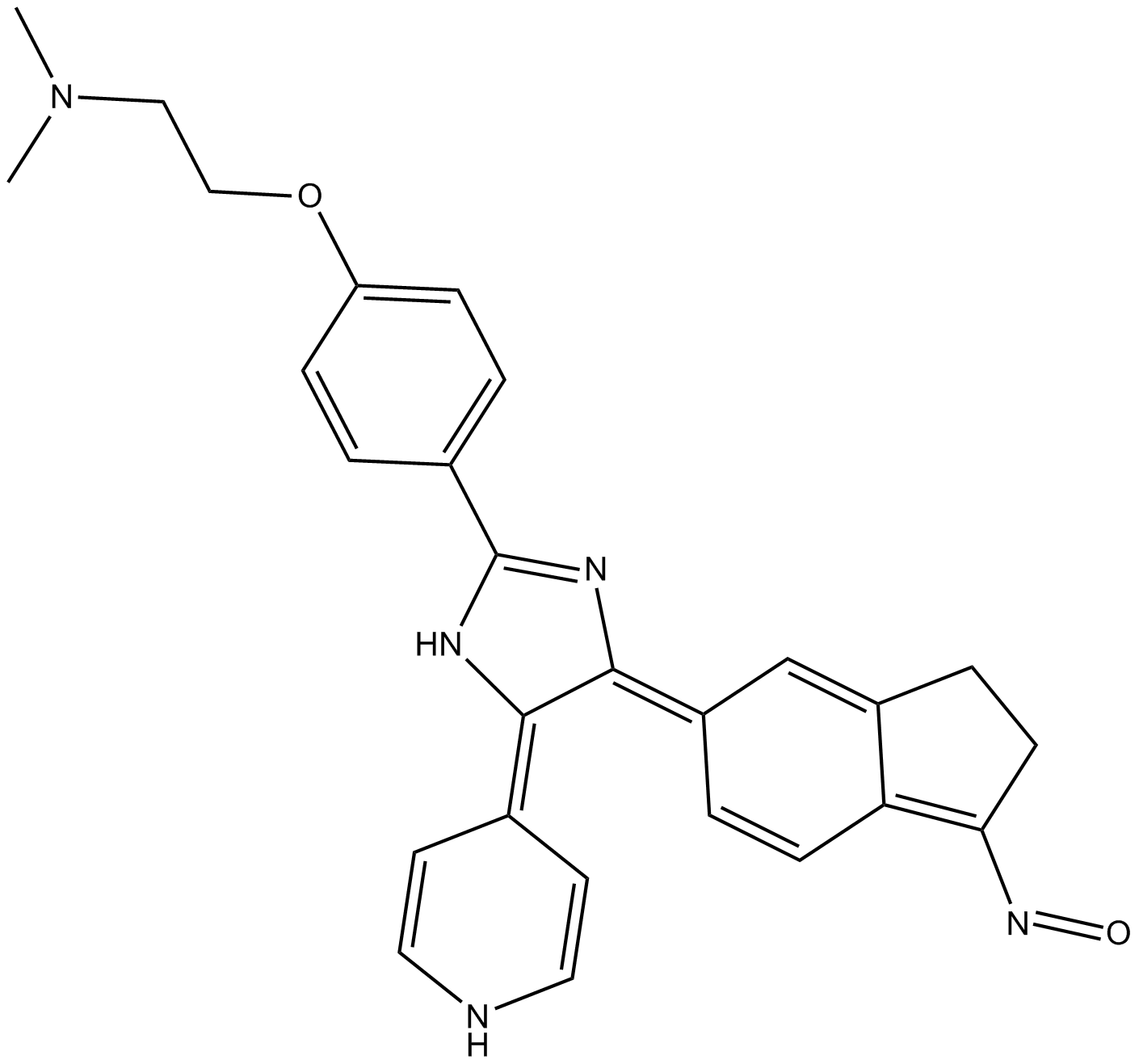 nd S bambuterol on the beta-2 receptors that regulate lipoprotein metabolism, Therefore, there is compelling hypothesis generating human data that selective beta-2 agonist may have beneficial effects on lipid metabolism.
Beta-2 agonism stimulates intracellular cAMP, which regulates a number of pathways involved in lipid and glucose metabolism. Sterol regulatory element-binding proteins (SREBPs) are major transcription factors regulating the biosynthesis of cholesterol, fatty acids and triglycerides (). SREBP is controlled by cAMP, which may explain why beta-2 agonism may affect LDL-c, HDL-c and triglyceride. In addition, cAMP stimulates ABCA1 upregulation, which results in HDL mediated cholesterol efflux from macrophages or hepatocytes ().
nd S bambuterol on the beta-2 receptors that regulate lipoprotein metabolism, Therefore, there is compelling hypothesis generating human data that selective beta-2 agonist may have beneficial effects on lipid metabolism.
Beta-2 agonism stimulates intracellular cAMP, which regulates a number of pathways involved in lipid and glucose metabolism. Sterol regulatory element-binding proteins (SREBPs) are major transcription factors regulating the biosynthesis of cholesterol, fatty acids and triglycerides (). SREBP is controlled by cAMP, which may explain why beta-2 agonism may affect LDL-c, HDL-c and triglyceride. In addition, cAMP stimulates ABCA1 upregulation, which results in HDL mediated cholesterol efflux from macrophages or hepatocytes ().